Known as ‘forever chemicals,’ per- and polyfluoroalkyl substances (PFAS) are compounds that impact our lives daily. Waterproof, durable, and long-lasting, PFAS are commonly used to repel water and grease. The chemicals are found in Teflon™-coated pans, fast food wrappers, and even in the firefighting industry.

While PFAS have many useful applications, they are proven to be highly toxic, causing harm to the human body and ecosystems. And now, they are embedded in society.
Even though PFAS are extremely prevalent in today’s environment, there are few ways to clearly identify them. Joe Charbonnet, Iowa State University Department of Civil, Construction and Environmental Engineering researcher and assistant professor, is creating a way for scientists around the world to communicate the characteristics of compounds they come across using one of the main PFAS identification methods – high-resolution mass spectrometry.
“PFAS are seen in ScotchGard™, popcorn bags and similar finished surfaces. They all consist of a carbon bonded to multiple fluorine atoms as part of their chemical structure,” Charbonnet said. “This project is about developing a more clear way for scientists to communicate when they have discovered a new PFAS. These are a big concern in the world right now because they are toxic, they last for a long time, and they build up in our bodies, making them a very urgent area of research.”
There are over 6,000 known types of PFAS. Sometimes the structure of the PFAS makes it easy to identify. But sometimes, it’s a little more complicated.
“It can be very clear – occasionally scientists can say, ‘I know exactly what the structure of the compound is’ in one test, and those are the easy cases,” Charbonnet said. “But that’s not the case for most.”
Using a strictly pure “example” molecule called a certified analytical reference standard, the scientist can test the PFAS sampled to a different PFAS that has already been discovered. This is one of the clearest ways to confidently identify a compound: If the compounds match, it is almost certain that the PFAS the scientist brought in is the same compound in the lab. But of the over 6,000 PFAS in existence, less than 100 of them have a certified exact match to compare to. Because of the high reliability, but low availability of this comparison method, scientists have been developing new ways to identify compounds that appear to be PFAS.

One of the additional methods of identifying a potential PFAS is called high-resolution mass spectrometry, which is a tool used to help determine the structure and qualities of a chemical. Some molecules can be made of the same atoms, but when arranged differently, become different molecules. The high-resolution mass spectrometer breaks apart molecules into smaller pieces, allowing scientists to see which atoms are bonded together. Seeing certain atoms bond together gives scientists insight into the structure of the chemical, which can increase their certainty in identifying what type of PFAS it could be, if it is one.
“By fragmenting these molecules, we can learn what their structure is. And the better that fragmentation is, the more certain you can be that it is the chemical that you think it is,” Charbonnet said.
Since mass spectrometry is a highly efficient method for identifying compounds, it is important to make sure the scientists using this method are all recording their data in the same way. Charbonnet created a solution for this exact issue – a framework to help scientists walk through data they gathered from mass spectrometry and structure-matching studies. Charbonnet and colleagues from three continents designed a table laying out the different results researchers can get from a high resolution mass spectrometry experiment. The table determines how much confidence a scientist can have in their PFAS identification, organized from most confident to least. The more boxes that are checked, the more confident the scientists can be that their proposed PFAS structure is accurate. Level one starts with the clearest method, a successfully matched structure using the reference matching method. Charbonnet compares the scale to nabbing a getaway car.
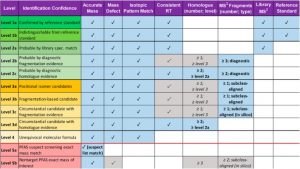
“A level five identification might be the molecular equivalent of saying, ‘the bank robber escaped in a blue sedan,’ while level one provides an exact molecular structure, something like, ‘the bank robber escaped in a blue 2008 Nissan Sentra with Nevada license plate ANF-53B and a broken left taillight.’ You’re much more likely to catch the bad guy,” Charbonnet said.
Confidence scales like Charbonnet’s have existed before, especially for spectrometry studies. But when PFAS started to be studied extensively, researchers found gray areas in the rules for characterizing the chemical.
“It’s really important that we talk clearly with each other when we say we found something new, or found something poorly characterized,” Charbonnet said. “Using this framework, everyone can understand how certain you are that you found what you found. Because there are some interesting tools that you can use with PFAS, we felt the need to more clearly define the confidence of the identification.”
“In the public space, we care a lot about PFAS, especially right now. These are compounds that we know are toxic, we know are harmful to humans and ecosystems, so we are concerned about ways to identify them and where they came from, and to get rid of them. So by communicating more clearly when we find these compounds, we become more aware of the types of chemicals that we are exposed to,” Charbonnet said. “This table helps to clarify the context for PFAS specifically, and how strong your evidence is when you use these types of tools.”
Charbonnet’s research was published this month in Environmental Science & Technology Letters, a prestigious journal highlighting environmental research.
