
Recent graduates in electrical engineering Josh Grindeland and John Etherington, and graduates in materials engineering Tommy Geske and Trent Borman proved that it is possible to extract electrical energy from waste heat at the ECpE Senior Design Demonstration on May 30.
The team had spent nine months prior designing and developing a device that uses a class of materials, known as liquid crystals, to generate pyroelectricity.
Pyroelectricity is observed in materials that undergo a change in local charge distribution upon change in temperature. Their device harnesses this atomic scale motion to harvest energy from local changes in the temperature.
The team made final preparations for their presentation by tuning the device and the data collecting software MatLab.
They were going to graph a live data stream to demonstrate that they had succeeded in extracting electrical energy from liquid crystals after applying arbitrary thermal fluctuations on their device.
Moving and grooving liquid crystals to generate electricity
Liquid crystals are a special polymer that undergoes a crystalline phase transformation near room temperature.
Geske says liquid crystals start out being randomly ordered. They have a built-in polarization, and at low temperature, this makes them want to align into an ordered structure when exposed to an electric field.
Changing the temperature causes this ordering to dissipate due to thermal vibrations in the system.
“When you start adding temperature, the liquid crystals begin to move more and more until they cross over our return point – also known as a Curie point – and they lose some of their crystallinity, or original order,” he explained.
This change in order causes a change in the liquid crystal’s internal energy.
The goal of the group’s project was to transform the energy associated with the change in temperature to the liquid crystal’s internal energy, which can become usable electrical energy.
Proof that the pyroelectric effect be used to generate energy
Starting with alligator clips, the team wired a 10mm by 10mm cell of liquid crystals to a circuit and microcontroller that were programmed to autonomously trigger changes in temperature and voltage.
The students then applied an electric field onto the liquid crystals and alternated it between a high and low voltage as they cycled the temperature by moving the device between a hot plate and an aluminum heat sink, as shown in the video.
“The larger the change between high voltage and low voltage, the greater the amount of net energy is produced,” Grindeland explained. The team alternated the electric field between 150 volts and 50 volts.
They used the software MatLab to graph a live data stream of the circuit’s activity, as shown near the end of the video.
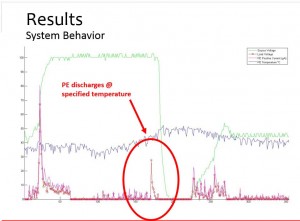
As shown in the photo, the green line represents voltage – the electrical field that was applied. The blue line represents temperature. The larger pink spikes are error points while the smaller pink spikes are noise.
The medium-sized red peak circled in the data demonstrates the project was a success.
Collaborative project fosters teamwork between engineering departments
Scott Beckman, assistant professor of materials science and engineering, and Sumit Chaudhary, associate professor of electrical and computer engineering, assembled and supervised the original team as part of a collaborative project between their departments.
The departments received $5,000 in funding from Iowa State alumnus Pete Onstad (BS’57 Electrical Engineering) for join senior design projects, after which the team began their efforts in August 2013. Onstad said he supports collaborative projects because they give students an opportunity to prepare themselves for work in the professional world.
“When engineering students graduate and go to work for corporations and companies in industry, they’ll most likely start off working in teams,” Onstad explained.
Geske said the project presented a number of challenges that the MSE and EE students overcame as a team. The most notable challenge was communication between the two engineering disciplines.
Grindeland explained that the team initially had a lack of background knowledge about what the pyroelectric device really was and how it worked. But with time and a lot of reading, they were able to get a better understanding of how the liquid crystals worked and apply that information to their project.
“It was a high-risk project, there was no guarantee that it would work,” Beckman said. “But this group of students pressed on and turned whiteboard concepts into a working device.”
All of the team members graduated from Iowa State this spring, but the research they’ve conducted has laid the groundwork for future senior design projects and potentially graduate research.