This story was originally published by Jorge Salazar with Scientific Computing.
New supercomputer models have come closer than ever to capturing the behavior of normal human heart valves and their replacements, according to recent studies by groups including scientists at the Institute for Computational Engineering and Sciences (ICES) at The University of Texas at Austin and the Department of Mechanical Engineering at Iowa State University.
The studies focused on how heart valve tissue responds to realistic blood flow. The new models can help doctors make more durable repair and replacement of heart valves.
“At the core of what we do is the development of new material models that are much more structurally and biologically informed and can actually integrate mechanisms of failure and remodeling, growth and adaptation to altered forces that go on,” study co-author Michael Sacks said. Sacks is the W.A. Moncrief Endowment Simulation-Based Engineering Science Chair at ICES, and professor of Biomedical Engineering, UT Austin.
ICES has a long history with TACC. Since 2003, ICES has used supercomputers at the Texas Advanced Computing Center (TACC) to study turbulent flow. These recent studies that model the leaflet tissue-blood flow interactions through replacement human heart valves have used millions of CPU hours on the Stampede, Lonestar and Maverick supercomputers at TACC.
“One of the big advantages to being here at ICES is that we have direct access to TACC facilities,” Sacks said. “These jobs run multiple processors over a whole wide range to try and get it working over the cardiac cycle. It’s very computationally intensive. The TACC resources are absolutely essential to these models. TACC facilities also allow us to concentrate on the technical and scientific problems at hand, rather than developing and maintaining hardware.”
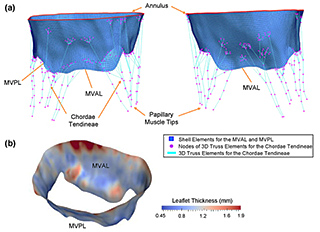
Two studies out in May 2015 centered on the leaflet cells of the mitral valve, the largest of the four heart valves. After a heart attack, the weakened heart wall will often cause the mitral valve to regulate, so that the normal one-way flow of oxygen-rich blood from the lungs between the left atrium into the left ventricle, where it is pumped out to the rest of the body.
On average, the heart beats about 2.5 billion times in a human life. Each pulse puts wear and tear on the constituent cells of the mitral valve leaflet. What’s more, the valve tissue remodels itself in response to the strain of its gatekeeping.
A May 2015 study funded by the National Institutes of Health (NIH) and published in the Journal of Theoretical Biology simulated deformations at the cellular level within the anterior leaflet tissue of mitral valve. The science team combined experimental work with computation, stretching heart tissues under a microscope to provide critical data to model the mitral valve leaflet.
Heart valve tissues are built like a four-layer cake of fibrous proteins and cells. One of the main findings showed that each layer of the mitral valve tissue causes the cells within the layer to stretch differently to the relentless pumping of blood. Since the cells are the “maintenance workers” of the valve and respond to the working demands on the valve by being stretched in differing ways, this finding can help us model changes in the self-maintenance of the heart valve that lead to valve failure.
“The computational demands result primarily from the large deformations that occur in the native valve, resulting in the need for advanced material models that are computationally very demanding,” Sacks said. “There’s also very complex geometries,” he added, “when valve leaflets ‘contact’ and come together.”
Sacks and colleagues addressed these challenges in another study funded by the NIH and released May 2015 in the journal Biomechanics and Modeling in Mechanobiology. In it, they took what they learned about the architecture of actual tissue fibers of the mitral heart valve and applied it to a big-picture model that simulated function at the organ level.
The point of all this research, said Sacks, is to develop models on how to improve the durability of surgically-repaired heart valves, which studies show can fail within as little as three to five years after repair.
“One of the major challenges there result from regurgitation of the valve,” Sacks said. Some blood leaks back across the mitral valve into the left atrium in a medical condition called ischemic mitral regurgitation.
“What we’re trying to do is develop models that can predict how a particular surgical technique can extend repair durability in a patient-specific manner,” Sacks said. “Ultimately, this gives surgeons tools to develop and improve surgical procedures.”
“This involves a number of different components, including the use of what’s called an annuloplasty ring,” Sacks said. “This ring is surgically implanted so that the valve no longer regurgitates, but different shapes induce differing stresses, which are thought to affect repair durability.”
Beyond repairs, each year more than 280,000 people worldwide replace their diseased heart valves with artificial heart valves. But even the newest artificial heart valves, made from tissue versus the older mechanical versions, only last about 10 to 15 years.
“They’re fabricated from soft tissues that have been chemically processed and, therefore, represent a challenge in terms of both the material modeling and also the fact they’re in a blood-contacting environment,” Sacks said. He added that there’s also the significant pressure from blood on the aortic heart valve that further challenges scientists’ ability to model them.
The stakes are high to model and improve artificial heart valves. “If you could make a valve that would last even three to five years longer, on average compared to the current valves, that would corner the market,” Sacks said. “It would have a huge clinical impact.”
Sacks and colleagues report their progress on modeling the aortic heart valve in a study funded by the National Heart, Lung, and Blood Institute (NIH funds) and the Food and Drug Administration, published February 2015 in the journal Computer Methods in Applied Mechanics and Engineering.
In it, the science team developed what they call a geometrically flexible technique for computationalfluid–structure interaction. “It’s very challenging, because of the mathematics handling how a fluid flows and how a solid deforms are fundamentally different,” Sacks said. “Coupling them is at the cutting edge of computational mechanics.”
Study co-author Ming-Chen Hsu has been working on artificial heart valves since 2012. Formerly a post-doc at ICES, Hsu was a post-doc with Thomas Hughes and Sacks, and is currently an assistant professor in the Department of Mechanical Engineering at Iowa State University.
“What we found is that it’s extremely challenging to consider the coupling between the blood flow and the heart valve structure using the current simulation frameworks. I think one of the most important contributions in this work is that we developed a computational technology that is able to directly tackle this issue,” Hsu said.
Hsu said that important “quantities of interest” such as mechanical stress emerge from computer models, something that is challenging to get today from even the most sophisticated experiments with live tissue.
“What’s new in this work is that we combined everything,” Hsu said. “We start from the design of the leaflet, use that directly into our analysis framework and perform fluid structure coupling study, and then investigate the mechanical stresses. It involves the idea of isogeometric analysis that has been very popular in the past 10 years. This framework can streamline everything in performing these kind of studies.”
Like the work with the mitral valve, the computational modeling effort with artificial aortic heart valves is all about durability, according to Hsu. “The impact this will make is, by understanding the interaction between the blood flow and heart valve leaflets, that we may be able to better design the material and the shape of the leaflet so that we can elongate the lifespan of the bioprosthetic heart valve. Hopefully, with the improved design, the patient doesn’t have to go through the painful process of heart valve replacement surgery every 10 to 15 years,” Hsu said.
What’s computationally challenging about heart valve leaflet, said Hsu, is it’s a very thin and flexible structure. “Computationally, especially for fluid–structure interaction, it’s very challenging to have a robust algorithm to handle these structural motions. What’s more, the material response is highly nonlinear. And when the strain is higher, the tissue will stiffen.”
There’s also lots of contact between leaflets as the heart valve opens and closes, which vex efforts to model them. “In computational mechanics, contact problem is a huge, challenging area to be addressed,” Hsu said.
The blood flow inside the heart is usually somewhere between smooth laminar to rough turbulence, which makes it hard to simulate, especially in the presence of the leaflets. “The most important thing is you want your method to be robust,” said Hsu. “We want to study the long-term fatigue of these materials and designs. You don’t want to be able to simulate just one cycle. You may want to simulate as many cycles as possible and see how these quantities change over time. It’s not just accuracy, but the robustness that is extremely challenging in the FSI framework.”
Sacks sees this research impacting just about anybody who’s going to get the next generation of heart valve replacements, especially the percutaneous, or non-surgically replaced artificial valve.
“Essentially, what you want to do is be able to engineer these devices the same way one engineers your iPhone,” Sacks said. “At the end of the day, the goal is to be able to produce a more effective therapy. That’s it. We’re using these modeling techniques to be able to do that.”