Iowa State engineers are exploring new approaches to produce biobased chemicals and fuels through electrochemistry.

Molecules derived from biomass are typically highly oxygenated, and require chemical transformations before they can be used as fuels or for the production of chemical products. One common transformation is hydrogenation – that is the addition of hydrogen – which is conventionally done using hydrogen gas (H2), high temperatures and pressures, and expensive catalysts such as platinum or palladium. However, Wenzhen Li – Richard Seagrave Associate Professor of Chemical and Biological Engineering – and coworkers are studying an alternative process in which hydrogenations can occur at mild conditions by applying a small electrical voltage to metal electrodes.
Electrocatalytic hydrogenation
The process is called electrocatalytic hydrogenation (ECH), and Li says it has many advantages over traditional methods. In ECH, hydrogen atoms are generated electrochemically from water, therefore avoiding the kinetic barriers and challenges of H2 activation. This means that the reactions can be performed without the need for external H2 supply, at room temperature and ambient pressure, and on inexpensive metal electrodes. Moreover, because the reactions are driven by electricity, the energy input can potentially be supplied from renewable sources such as wind and solar power.
Insight leads to tunability
A key challenge of ECH is to achieve high selectivity and electron efficiency to the target products. For example, an undesired reaction that may occur is the coupling of two substrate molecules to form dimer products. Additionally, water, which is used as the solvent, may react at the electrode to generate H2 gas, resulting in lower efficiency for ECH and higher electricity demand.
“There are big understanding gaps about the actual mechanisms occurring at the metal surface, and little is known about the relationship between reaction conditions and selectivity,” said Li. His recent research focuses on distinguishing different mechanisms occurring during the ECH of furfural, a molecule derived from sugars. For this reaction, an inexpensive and Earth-abundant metal, copper, is used as the electrode material to selectively generate furfuryl alcohol, an important industrial chemical, or 2-methyfuran, a promising biofuel. “I am very proud of our work because we acquired new understanding of electrochemical reduction mechanisms by cleverly combining various experimental tools and sophisticated techniques,” said Li.
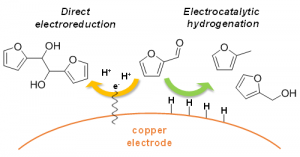
Xiaotong Chadderdon, a Ph.D. candidate in Li’s research group, explained “the insight gained about the different surface processes allowed us to rationally choose conditions, such as the electrical voltage and electrolyte pH, in order to maximize the desired reactions.”
Their findings were published in a recent issue of the Journal of the American Chemical Society, in an article titled “Mechanisms of Furfural Reduction on Metal Electrodes: Distinguishing Pathways for Selective Hydrogenation of Bioderived Oxygenates” (DOI: 10.1021/jacs.7b06331). Xiaotong is a co-first author of the article along with David Chadderdon, who is also a Ph.D. candidate in Li’s group. Additional co-authors are Jean-Phillipe Tessonnier, Assistant Professor of Chemical and Biological Engineering; Dr. John Matthiesen, a Ph.D. graduate of Tessonnier’s group; Dr. Jack Carraher. a former post-doctoral researcher in Tessonnier’s group; and Yang Qiu, a Ph.D. candidate in Li’s group. Li and Tessonnier groups worked closely together on the project, which was supported by funds from the U.S. National Science Foundation.
Toward a sustainable future
Professor Li has high hopes that electrochemistry will be a key part of sustainable chemical and fuel production in the future.
“The electrochemical approach is unique, because it takes advantage of both renewable carbon in the form of biomass-derived feedstocks and renewable electricity, which is expected to become more abundant and inexpensive in the future,” said Li. “Our recent findings are not only applicable to furfural, but can potentially be extended to the ECH of different biomass-derived chemicals and to other challenging electrochemical reactions.”
He also foresees opportunities in the future to replace copper with light-absorbing semiconductor electrodes – which could be used to harness energy directly from sunlight to drive electrochemical reactions.